Your Specific heat capacity experiment images are available in this site. Specific heat capacity experiment are a topic that is being searched for and liked by netizens now. You can Download the Specific heat capacity experiment files here. Find and Download all free photos and vectors.
If you’re searching for specific heat capacity experiment images information linked to the specific heat capacity experiment interest, you have pay a visit to the right site. Our website frequently gives you suggestions for seeing the highest quality video and image content, please kindly surf and locate more informative video content and graphics that match your interests.
Specific Heat Capacity Experiment. This is an instructional video for Unit 1 VCE Physics. This means that it takes 4 Joules of energy to raise 1 gram of water 1 degree Celsius. Solids Specific heat capacity of solids is important to understand in lots of applications that deal with heat energy and changes in temperature. A diagram of the apparatus used in an experiment to find the specific heat capacity of a metal block.
 Heat Capacity Of Water Vs Heat Capacity Of Oil Science Project Education Com Cool Science Fair Projects Science Fair Projects Water Science Experiments From pinterest.com
Heat Capacity Of Water Vs Heat Capacity Of Oil Science Project Education Com Cool Science Fair Projects Science Fair Projects Water Science Experiments From pinterest.com
Thespecific heat capacity of sample 2 was 0 kJkg-1 K-1. At that time it was found that the specific heat at constant volume Cv of a substance is defined to be its heat capacity per unit mass when all changes are made at a fixed volume. Mr Rees shows you how to measure the SHC of a material using the graph method—–0000 Experiment setup0108 SHC equat. Specific Heat of a Metal Purpose. Solids Specific heat capacity of solids is important to understand in lots of applications that deal with heat energy and changes in temperature. The study of specific heat falls under the category of Thermochemistry which.
This experiment allows you to control the electrical heating power applied to a choice of six different materials and measure the rate at which the sample temperature changes.
Thespecific heat capacity of sample 2 was 0 kJkg-1 K-1. References Theory of Heat Maxwell James Clerk page 57-67 Westport Conn Greenwood Press 1970. The actual value for the specific heat capacity of aluminium is 900 JkgC. Specific Heat of a Metal Purpose. Let us note that if we know the specific heat capacity c of a substance of mass m which is heated cooled by Δ t the heat Q supplied to taken out of the substance can be expressed as. We know the specific heat capacity of water is 4200JKgK.
 Source: pinterest.com
Source: pinterest.com
Energy mass x specific heat capacity x temperature change This will be the same energy change as the metal block. This is an instructional video for Unit 1 VCE Physics. For a body of. Heat 250 mL of water in a 400-mL beaker until it is boiling gently. C which is a large value compared to other sub-stances.
 Source: pinterest.com
Source: pinterest.com
The heat capacity of an object C is defined as the amount of heat that must be added to the object to raise its temperature by 1 K or 1 C. Specific Heat Capacity Introduction This experiment is designed to introduce you to the concept of specific heat capacity and how you can measure it for different substances. Experiments Variables and Graphs. Every substance has a characteristic value. While the water is heating determine and record the mass of a clean dry 50-mL beaker to the nearest 001 g.
 Source: pinterest.com
Source: pinterest.com
From doing the above experiment we obtained the following data. The specific heat of a substance is the heat capacity per unit mass. In this experiment we will determine the specific heat capacity of a metal and compare it to an accepted value. The water equivalent of a body is the mass of water which would require the same amount of heat as the body in order to raise the temperature through one. This experiment is an extremely quick and relatively precise specific heat capacity test for a solid sample.
 Source: br.pinterest.com
Source: br.pinterest.com
1 This is not to be confused with heat capacity which is the amount of energy required to raise the temperature of a substance in varying amounts by 1 degree Celsius. We know the specific heat capacity of water is 4200JKgK. This experiment allows you to control the electrical heating power applied to a choice of six different materials and measure the rate at which the sample temperature changes. The actual value for the specific heat capacity of aluminium is 900 JkgC. The water equivalent of a body is the mass of water which would require the same amount of heat as the body in order to raise the temperature through one.
 Source: pinterest.com
Source: pinterest.com
Experiments Variables and Graphs. By dividing out the mass one gets the specific heat capacity c or simply the specific heat. Energy mass x specific heat capacity x temperature change This will be the same energy change as the metal block. The specific heat is essentially a measure of how thermally insensitive a substance is to the addition of energy. The purpose of this experiment was to find the specific heat capacity of the materialby measuring the temperature change when the metal was placed inside thecalorimeter.
 Source: pinterest.com
Source: pinterest.com
The water equivalent of a body is the mass of water which would require the same amount of heat as the body in order to raise the temperature through one. Thus in this experiment we use as the unit of heat not. Aluminium which is what the calorimeter is made from has a specific heat capacity of 880-937 Jkgk at a temperature of 273-373K 0-100 degrees Celsius. The actual value for the specific heat capacity of aluminium is 900 JkgC. In another system of units the specific heat of water has the value 100calorieg C.
 Source: pinterest.com
Source: pinterest.com
The purpose of this experiment was to find the specific heat capacity of the materialby measuring the temperature change when the metal was placed inside thecalorimeter. Questions Have Been Asked. The water equivalent of a body is the mass of water which would require the same amount of heat as the body in order to raise the temperature through one. Mass of aluminium without water 00323 kg. The amount of heat Q gained or lost by a substance is.
 Source: pinterest.com
Source: pinterest.com
The actual value for the specific heat capacity of aluminium is 900 JkgC. MT2 T1 Q M C c 1 The units of specific heat in SI are J kg C. The specific heat of a substance is the amount of heat needed to raise the temperature of 100kg of the substance 100 C. In the SI system water has a specific heat of 4184Jkg. The water equivalent of a body is the mass of water which would require the same amount of heat as the body in order to raise the temperature through one.
 Source: pinterest.com
Source: pinterest.com
This experiment is an extremely quick and relatively precise specific heat capacity test for a solid sample. The purpose of this experiment was to find the specific heat capacity of the materialby measuring the temperature change when the metal was placed inside thecalorimeter. We know the specific heat capacity of water is 4200JKgK. Experiments Variables and Graphs. The heat capacity of an object C is defined as the amount of heat that must be added to the object to raise its temperature by 1 K or 1 C.
 Source: in.pinterest.com
Source: in.pinterest.com
1 This is not to be confused with heat capacity which is the amount of energy required to raise the temperature of a substance in varying amounts by 1 degree Celsius. Experiments Variables and Graphs. The water equivalent of a body is the mass of water which would require the same amount of heat as the body in order to raise the temperature through one. Specific heat capacity energymass x temperature change. C which is a large value compared to other sub-stances.
 Source: pinterest.com
Source: pinterest.com
The specific heat of a substance is the heat capacity per unit mass. From doing the above experiment we obtained the following data. Measure the mass of the metal block using an electronic balance. MT2 T1 Q M C c 1 The units of specific heat in SI are J kg C. This experiment allows you to control the electrical heating power applied to a choice of six different materials and measure the rate at which the sample temperature changes.
 Source: pinterest.com
Source: pinterest.com
Heat 250 mL of water in a 400-mL beaker until it is boiling gently. The purpose of this experiment was to find the specific heat capacity of the materialby measuring the temperature change when the metal was placed inside thecalorimeter. By dividing out the mass one gets the specific heat capacity c or simply the specific heat. C which is a large value compared to other sub-stances. In another system of units the specific heat of water has the value 100calorieg C.
 Source: pinterest.com
Source: pinterest.com
Mass of aluminium with water 01477 kg. References Theory of Heat Maxwell James Clerk page 57-67 Westport Conn Greenwood Press 1970. 1 Q c m Δ t In this experiment the water is heated by electric current passing through a heating coil. The specific heat capacity of sample 1 was 0 kJkg-1 K-1. Mass of aluminium with water 01477 kg.
 Source: pinterest.com
Source: pinterest.com
The actual value for the specific heat capacity of aluminium is 900 JkgC. The amount of heat Q gained or lost by a substance is. C which is a large value compared to other sub-stances. In a calorimetry experiment heat is transferred from one object to another inside an insulated container called a calorimeter. Specific Heat of a Metal Purpose.
 Source: es.pinterest.com
Source: es.pinterest.com
Specific Heat of a Metal Purpose. Historically specific means referred to water and the measurements done in this experiment are referred to the specific heat of water. Specific heat capacity energymass x temperature change. The specific heat capacity can then be calculated using. The actual value for the specific heat capacity of aluminium is 900 JkgC.
 Source: pinterest.com
Source: pinterest.com
In this experiment we will determine the specific heat capacity of a metal and compare it to an accepted value. At that time it was found that the specific heat at constant volume Cv of a substance is defined to be its heat capacity per unit mass when all changes are made at a fixed volume. Thespecific heat capacity of sample 2 was 0 kJkg-1 K-1. Energy mass x specific heat capacity x temperature change This will be the same energy change as the metal block. The specific heat capacity can then be calculated using.
 Source: pinterest.com
Source: pinterest.com
The actual value for the specific heat capacity of aluminium is 900 JkgC. In a calorimetry experiment heat is transferred from one object to another inside an insulated container called a calorimeter. Thus in this experiment we use as the unit of heat not. Connect an Ammeter power supply and immersion heater in series. Questions Have Been Asked.
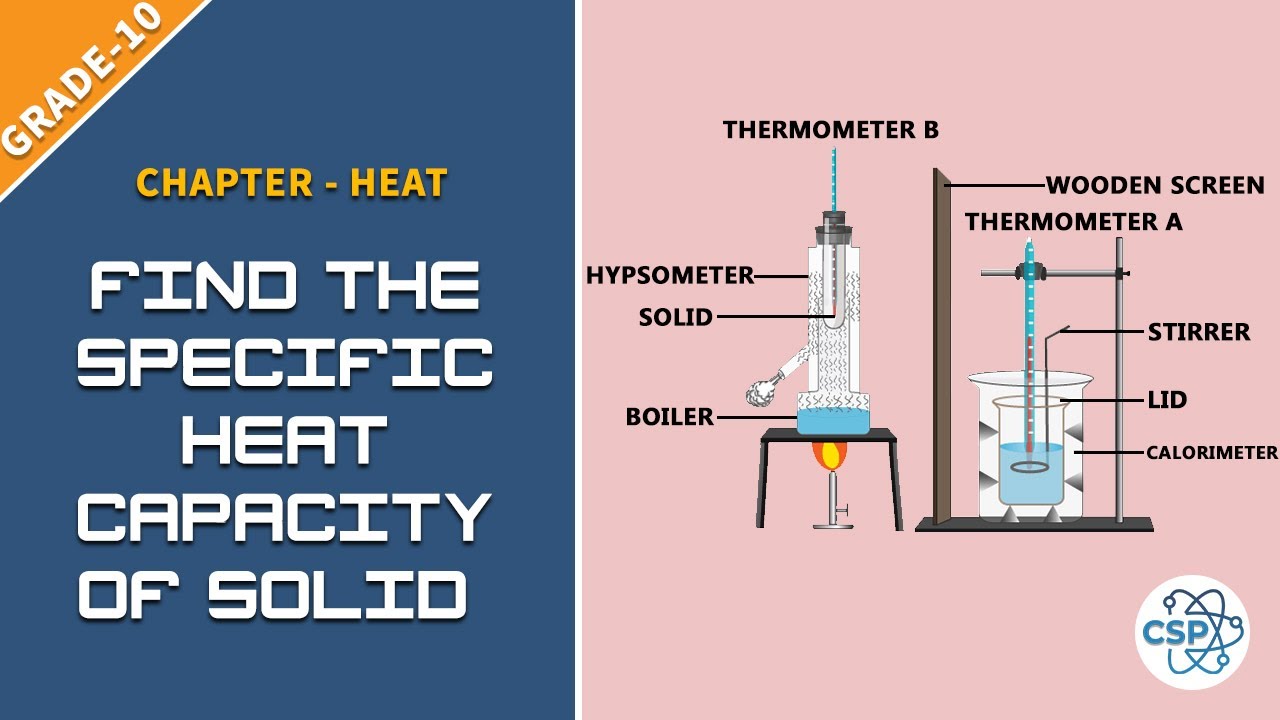 Source: in.pinterest.com
Source: in.pinterest.com
Measure the mass of the metal block using an electronic balance. The specific heat of a substance is the amount of heat needed to raise the temperature of 100kg of the substance 100 C. In this experiment we will determine the specific heat capacity of a metal and compare it to an accepted value. The calculated value does not match exactly but it is in the correct order of magnitude. This means that it takes 4 Joules of energy to raise 1 gram of water 1 degree Celsius.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title specific heat capacity experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






